Roestvast staal in de voedingsmiddelen- en drankenindustrie (EN)
Reprinted from Euro Inox with permission.
Author: Eric Partington, Fairford (UK)
Title Photos: Centro Inox, Milan (I), Koninklijke Grolsch, Enschede (NL), Packo Inox, Zedelgem (B)
Contents
1 General scope and objectives
2 Background
3 Why stainless steel
4 Which stainless steel
5 How to fabricate stainless steel
6 Surface finish
7 Design principles
8 Conclusion
9 European standards
10 References
11 Where to go for help
12 Appendix
1 General scope and objectives
This brochure offers an overview of the versatility of stainless steel as the material of choice in the European (and, indeed, global) Food and Beverage Industries. It describes the characteristics of the five main groups of stainless steel and indicates appropriate applications for each. It discusses the techniques for manipulating stainless steels and the importance of both a smooth surface finish and correct, hygienic design. However, it cannot encompass every aspect of the selection and application of stainless steels and so it includes helpful references to direct the reader to sources of specialist advice.
2 Background
There is a wide range of stainless steels, each of which offers its own unique portfolio of corrosion resistance, mechanical strength, formability and weldability. Using the most appropriate stainless steel, preparing it correctly and maintaining it well can ensure long, trouble-free service at minimum life-cycle cost.
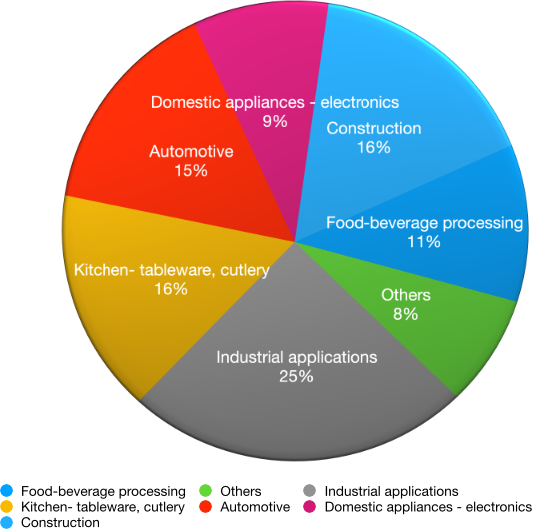
3 Why stainless steel
Long ago stainless steels established them- selves as the materials of choice for the construction of almost all food-processing and storage equipment. But why? What attributes make stainless steels so suitable?
First and foremost must be their corrosion resistance. We are all familiar with how clean and bright stainless steels remain under normal atmospheric conditions but it is this general inertness which also makes them ideal for food contact. After all, if there is no measurable chemical reaction between the stainless steel and the food, not only will the material remain pristine but so will the food, untainted by metallic constituents or corrosion products.

In Europe, the Framework Regulation (EC) 1935/2004 [1] specifically requires that "food contact materials, under normal or foreseeable conditions of use, do not transfer their constituents to foods in quantities which could endanger human health or bring about an unacceptable change in the composition of the food or a deterioration in its organoleptic characteristics". This regulation may be further specified in national regulations; e.g. [2].
Grade 1.4301 (AISI 304) — the stainless steel with which we are most familiar because of its wide-spread use for domestic cutlery and hollowware — is used so extensively in food and beverage preparation precisely because it remains inert in so many food environments. And it is tolerant of the powerful detergents which may be required to keep the plant clean. In fact, stainless steel is rapidly replacing other metallic materials in food processing and catering equipment.
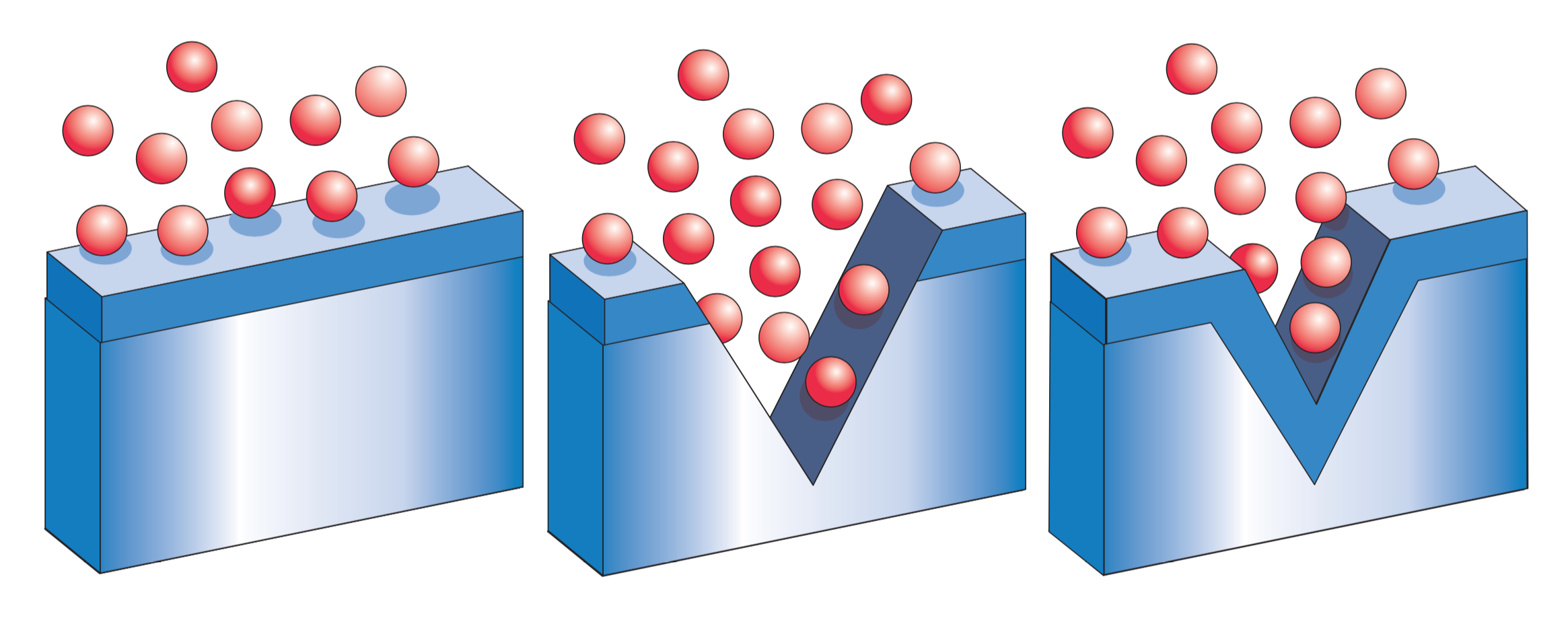
This corrosion resistance comes from an invisible, protective film of chromium-rich oxide which stainless steels containing 10.5% or more of chromium form spontaneously in the presence of oxygen or oxygenated water. Even if it is physically or chemically damaged, this film will very rapidly repair itself once the source of the damage is removed and the surface is exposed to oxygen again.
But, in addition to this inertness (which offers the added advantage of non-toxicity), stainless steels have many more properties which make them ideal choices for foodprocessing plant and equipment. It is easy to create smooth, non-absorbent surfaces on stainless steels and the inherent hardness of this family of materials helps to keep those surfaces smooth. The relationship between the roughness of a food-contact surface and the ease with which foods will adhere to it (and also the difficulty of their removal) is well-documented [3], and so a surface which will remain smooth for a long period of time will resist the accumulation of bio-films which can present a hygiene hazard. It will therefore remain easy to maintain to a high standard of hygiene.
Stainless steels are tolerant of the wide range of temperatures frequently encountered in the production of foods, from cooking to freezing, and they resist thermal shock — a rapid and significant change of temperature — very well.
Most importantly, the physical properties of stainless steels make it easy to construct from them plant which can process foods effectively and economically and to design with them equipment which can easily be cleaned and maintained, often without the need for dismantling (Cleaning in Place). They are strong. They can be formed into the necessary shapes for food processing, transportation and storage, and they are readily welded (see section 5 below). They resist impact, fatigue, wear, abrasion and erosion well.
Furthermore, HACCP (Hazard And Critical Control Point) analysis requires that the operators of any commercial process, including food processing and packaging, should actively look for potential threats to health, safety and hygiene. A 'threat' is defined as anything from infection resulting from poor design or lack of hygiene, through the degradation of materials by erosion or corrosion, to component failure by fracture or fatigue. HACCP systems must now consider the possibility of materials contaminating the foods with which they are in contact. In any state of the EU, competent national authorities' inspectors can demand to see HACCP analyses to deter- mine if all possible risks really have been assessed and appropriate action taken. Of course, as with any material, the selection of the most appropriate grade of stainless steel and the optimum surface finish for the intended conditions of use, together with the application of good design, fabrication and maintenance practices is essential, and these are discussed later in this brochure.
The reliability and longevity of stainless steels also contribute significantly to their favourable Life-Cycle Costs. But even when a piece of stainless steel equipment has reached the end of its useful life, the stainless steel itself hasn't. Today, a newly-manufactured stainless steel object will have an average recycled content of about 60%1.
And a feature particularly important when presenting food either in the retail outlet or in the domestic environment is that stainless steels are pleasing in appearance.

The popularity of stainless steels in the food industry is due to a combination of practicality and aesthetics.
1 ISSF figure. See also CD-ROM The Recycling of Stainless Steel Luxembourg: Euro Inox 2004
4 Which stainless steel
Just as 'steel' is iron which contains a controlled amount of carbon, so 'stainless steel' is a steel which contains a controlled amount of chromium. However, 'stainless steel' is not a single material but a family of over 200 iron-carbon-chromium alloys. Stainless steels are steels which contain a maximum of 1.2% carbon and a minimum of 10.5% chromium. Of course, this does not mean that every grade of steel with more than 10.5% chromium will resist staining in every operating environment. If the operating conditions are particularly aggressive some grades of stainless steel may suffer corrosion and so a grade which has more chromium, or which has additions of other elements such as nickel, molybdenum, nitrogen and copper, may be required because of its greater resistance to a particular environment or to a particular type of corrosion. Some additions may make the stainless steel more easily formed or machined or welded, which will make the fabrication of equipment easier, or they make the material tougher so that it is more durable. However, in doing so, they may reduce another property such as corrosion resistance and they may also add to the cost of the material. It is therefore important to define the environment in which an item of stainless steel equipment is to operate, to appreciate the fabrication processes which will be involved in its construction and to understand the effects which each alloying element will have on the material's properties. This will help to select a grade of stainless steel which is suitable, but not over-specified, for that particular application. Reference [4] offers an introduction to the metallurgy and the corrosion resistance of stainless steels and Euro Inox publishes tables of the properties of a wide range of stainless steels in coil, sheet or plate form [5].
The 'simplest' stainless steels are the iron-carbon-chromium alloys and these fall into two groups.
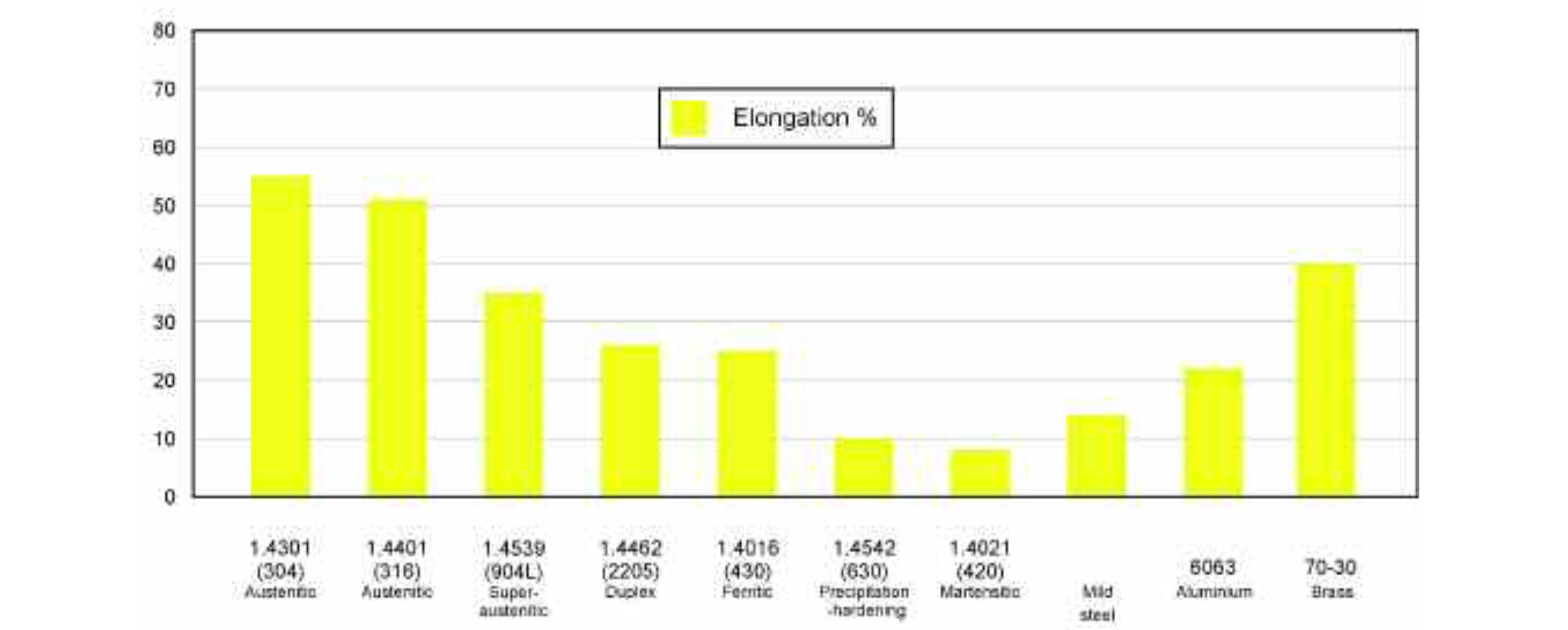
The first group is the 'martensitic' stainless steels. These contain only about 13% chromium (and so they are the least expensive stainless steels) but they have high levels of carbon (even up to 1%). Whilst the high level of carbon in the martensitic stainless steels makes them difficult to form and weld, it also makes them very hard and strong, and heat treatment can make them even harder. These stainless steels (sometimes referred to just as 'martensitics') are ideal where the environment is not particularly aggressive, but resistance to wear is important. Grade 1.4125 (AISI 440C) contains 1% carbon and is extremely hard and so it is used for the wearing parts of pumps. Grade 1.4021 (AISI 420) contains a minimum of 0.15% carbon and is ideal for knife blades. Grade 1.4116 contains a minimum of 0.45% carbon and is used for superior kitchen knives which will retain their sharpness even after prolonged use.

The other iron-carbon-chromium group is known as the 'ferritic' stainless steels and these will typically contain about 17% chromium and about 0.05% carbon. The significant characteristics of the ferritic stainless steels (often called just 'ferritics') are that they are magnetic. They are commonly used for household appliances such as dishwashers, refrigerators and pans. Grade 1.4016 (AISI 430) has acceptable corrosion resistance (especially to stress corrosion cracking) and is relatively inexpensive.

However, this ferritic is less easily formed or welded than the austenitic stainless steels (see below). For applications demanding significant forming or corrosion resistance, special ferritics can be selected. Where welding of ferritics is unavoidable, the use of one of the many grades which have been stabilised by the addition of titanium and/or niobium (such as 1.4509; AISI 441) is recommended, and stabilised ferritics are particularly suited to heater and burner components. Resistance to pitting corrosion will be enhanced by the addition of 2% molybdenum, and grade 1.4521 (AISI 444) will be used where there is a high level of chlorides in a neutral medium.
The addition of nickel to stainless steel offers valuable fabrication advantages (such as better formability and weldability) as well as improved corrosion resistance, and there are three groups of iron-carbon-chromium-nickel stainless steels. The first group is known as the 'austenitic' stainless steels or just 'austenitics'. An 8 – 12% nickel content makes them easy to form and yet tough. Their ductility means that they can be easily roll-formed, pressed and deep-drawn and their 18% chromium gives them an excellent defence against general corrosion. They are the most commonly-used stainless steels in the food and beverage industries.
Grade 1.4301 (AISI 304) is an austenitic stainless steel which contains approximate- ly 0.05% carbon, 18% chromium and a minimum of 8% nickel. It is used in a wide range of applications from brewing vessels to kitchen sinks to milk tankers. Where a component is to be produced by deep-drawing (as beer kegs are), the nickel content may be increased to 9% or more to improve the steel's formability.

As with the ferritic grade 1.4521, the addition of approximately 2% molybdenum to this austenitic stainless steel further improves its resistance to pitting corrosion. The 1.4401 (AISI 316) grade is AISI 304 with about 2% molybdenum added and it is particularly resistant to high levels of chloride or sulphur dioxide in the operating environment, making it suitable for the storage of white wines, salty foods and aggressive media such as the pectin used in jam-making.

The super-austenitic steels are tolerant of extremely aggressive conditions. During the production of soy sauce, for instance, the ingredients ferment in tanks for about six months, during which they produce a sauce rich in organic acids, amino acids and alcohols and with a pH of about 4.7 and a sodium chloride content of around 17%. High levels of chromium, nickel, molybdenum and nitrogen and a low carbon content confer to austenitic stainless steels superior corrosion resistance in a wide array of aggressive environments. Typical examples are grade 1.4539 (904L), which has a molybdenum content of over 4%, and grades 1.4547 (254 SMO) and 1.4529, both of which contain more than 6% molybdenum.

In very corrosive environments such as mustard and vinegar-making, cheese dairies or fish-canning plants, it may be necessary to use one of the second group of iron-carbon-chromium-nickel stainless steels — the 'duplex' steels. These have very high levels of chromium — 22% in grade 1.4462 (2205) and 23% in grade 1.4362 (2304) — and, in the case of 1.4462, about 3% molybdenum. Most duplex steels are also more expensive than the austenitics. They have a resistance to general corrosion similar to the austenitic stainless steels but much higher mechanical strength, in part due to an addition of about 0.15% of nitrogen. They also have a much better resistance to stress corrosion cracking than austenitic stainless steels (although not quite as good a resistance as the ferritic grades) and a resistance to crevice and pitting corrosion superior to the 1.4401 (AISI 316) austenitics.

Although the costs of the material content and manufacture of the third group of iron-carbon-chromium-nickel stainless steels are high, the 'precipitation-hardening' stainless steels combine the good corrosion resistance of the austenitic grades with the excellent mechanical properties of the martensitic steels. A steel such as grade 1.4542 (AISI 630) has additions of copper (which improves its resistance to reducing acids) and niobium (to help to reduce corrosion at welds).
5 How to fabricate stainless steel
As with all materials of construction, good 'housekeeping' is important when preparing to fabricate an engineering component from stainless steel. Sheet and strip can be purchased with a smooth surface finish (eg: 2B) and this should be protected, preferably by specifying that the mill applies an interleaf (possibly adhesive) paper or a coating which can be left on the surface during storage and much of the fabrication procedure.

Sheet and strip should ideally be stored upright in racks (never horizontally on the floor) so as to minimise the risk of damage from other components being dragged across them or from particles being trodden into their surfaces. Pipe can be ordered with protective end-covers and plastic wrapping which can be advantageous if the pipe is to be stored outdoors 2.

Cleanliness is essential as contamination can interfere with welding processes and can lead to crevice corrosion. Contaminants such as grease, oil, crayon markings and adhesive tape should be removed with a non-chlorinated solvent.
To avoid their contamination by carbon steels, the fabrication of stainless steel components should be carried out in an area separate from any similar work on carbon steels. It is important that particles of carbon steel do not become embedded in a stainless steel surface as these will not only corrode in a damp atmosphere, creating unsightly streaks of rust, but can also lead to pitting or crevice corrosion of the stainless steel in the indentation which is entrapping the carbon steel particle.
2 Detailed recommendations can be found in The Mechanical Finishing of Decorative Stainless Steel Surfaces (Materials and Applications Series, Volume 6), Luxembourg: Euro Inox, 2005
Embedded particles can be removed by pickling the surface with an acid, as described below, but prevention is always better than cure 3. Wire brushes should be therefore made from stainless, not carbon, steel. Layout or cutting tables which are made from carbon steel should be covered with cardboard or plastic sheets to prevent contact between the two materials. Carbon steel slings and hooks, and the forks of lift-trucks, should have wooden or plastic guards.
3 Pickling and Passivating Stainless Steel (Materials and Applications Series, Volume 4), Luxembourg: Euro Inox, 2004

Stainless steels can be cut by cropping or with a cutting disc, by plasma jet or water jet. They cannot be cut by the normal oxy-acetylene or oxy-propane methods used for carbon steels, although a variation on oxy-propane cutting, introducing iron powder into the flame, can be highly effective and can cut sections up to 200 mm thick.
Austenitic stainless steels are amongst the most easily-formed of all engineering materials. However, they have greater springback than non-stainless steels and so, when attempting to form accurate shapes, this must be allowed for. They also workharden, which means that, as they are formed, their strength increases.
The process of welding two stainless steel components together will affect the mechanical properties and the corrosion resistance of both the joint itself and the area immediately adjacent to the weld, but there has been extensive publication of advice on the welding of stainless steels [6, 7, 8].
Essential for all welding is the good housekeeping described above and consideration of the following phenomena, all of which, with the correct techniques and procedures 4, can be controlled:
- During the welding process itself, the molten metal must be shielded from atmospheric oxidation by means of a gas or a slag or a vacuum in order to achieve and preserve the optimum corrosion resistance and mechanical properties in the joint.
- On either side of a weld-run, the parent metal will have been heated to a temperature approaching its melting point and these areas are known as Heat Affected Zones (HAZ). How the characteristics of the weld-metal and the HAZ will be affected will depend upon the composition of the stainless steel and the weld technique, including the use of filler metals, and on subsequent chemical treatments.
- During welding, chromium and carbon in the stainless steel can form chromium carbide particles, a process known as sensitisation. This reduces the chromium available to combine with oxygen and so form the chromium oxide passive layer which gives stainless steel its corrosion resistance.
- The weld metal itself may suffer from micro- fissuring — the development of tiny cracks.
- The temperature gradient across the weld can give rise to distortion of the component, and during cooling high stresses can be set up in the weld and HAZ.
4 Welding stainless steels (Materials and Applications Series, Volume 3), Luxembourg: Euro Inox, 2001
Martensitic stainless steels are weldable but will form a hardened zone adjacent to the weld, the hardness of which will depend upon the carbon content of the steel. However, this can be controlled by appropriate welding procedures. Martensitics have relatively low thermal conductivities, which can give rise to very steep temperature gradients and stresses high enough to cause cracking. Pre-heating and post-weld heat treatment may be required to prevent such cracking.
Ferritic stainless steels tend to suffer from sensitisation. Grades of ferritic steels which are 'stabilised' by the addition of titanium and/or niobium, however, suffer less from this as those elements both combine with carbon even more readily than chromium, forming titanium or niobium carbides and leaving the chromium available to form the passive layer. Heat treatment after welding may also be necessary if a ferritic steel is not to suffer from brittleness at room temperatures.
Austenitic stainless steels are readily welded by all fusion and resistance methods. They have a lower coefficient of thermal conductivity than carbon steels, which results in a tendency for heat to concentrate in a narrow HAZ beside the weld. They also have coefficients of thermal expansion about 50% greater than carbon steels and so will tend to warp or distort after welding if this is not controlled. Grades with high levels of carbon can suffer from sensitisation, although this can be avoided by the addition of titanium, as in grade 1.4541 (AISI 321), which stabilises the steel. Heating 1.4301 (AISI 304), which contains up to 0.07% carbon, into the range 980°C to 1180°C after welding and then rapidly cooling it will cause the carbide particles to redissolve and will also relieve the stresses caused by the welding which might otherwise lead to stress-corrosion cracking. However, modern steel-making techniques now make low-carbon grades of austenitic stainless steels such as 1.4307 (AISI 304L) or 1.4404 (AISI 316L) readily available and welds in these grades have much improved corrosion resistance in highly aggressive environments. The use of the correct fillerwire will prevent the cracking of either the weld metal or the base metal near to the weld to which austenitic stainless steels can be susceptible.
Precipitation-hardening stainless steels are readily welded, but because of the many combinations of weld procedure and heat-treatment available for them, it is advisable to seek specialist advice on their welding.
Duplex stainless steels have become more weldable with the controlled addition of nitrogen and the development of nickel- enriched filler metals which improve both ductility and toughness in the weld.
All welding of all grades must be followed by an effective 'post-weld clean-up'. The heat of welding will leave areas of oxidation (known as 'heat tint') near to the weld which will have a significantly reduced resistance to corrosion, and this must be restored by removing the tint, ideally entirely. 'Pickling' is the controlled corrosion of the surface and will remove this undesirable oxidation. The usual medium is 10% nitric/3% hydrofluoric acid in a bath at about 50°C. Alternatively, for very large components or after welding repairs to installed plant, for instance, a paste of this acid may be applied to the weld. Pickling will remove any embedded iron particles and other metallic contamination and will leave the surface bright and clean, allowing the natural formation of the chromium oxide passive layer given the presence of oxygen. The duration of pickling must, however, be controlled so that it only removes the heat tint — excessive pickling can lead to intergranular corrosion in the HAZ in unstabilised grades of stainless steel.

Recognising the difficulty of gaining access, AWS 5 D18.1:1999 [9] does allow a limited level of heat tint to remain inside pipework intended for food use. It states, however, that a weld surface shall not contain excessive oxidation and considers that oxidation indicated by a discolouration greater than straw or light blue, as shown on the photograph as example 5, is unacceptable in the as-welded condition.
5 AWS: American Welding Society
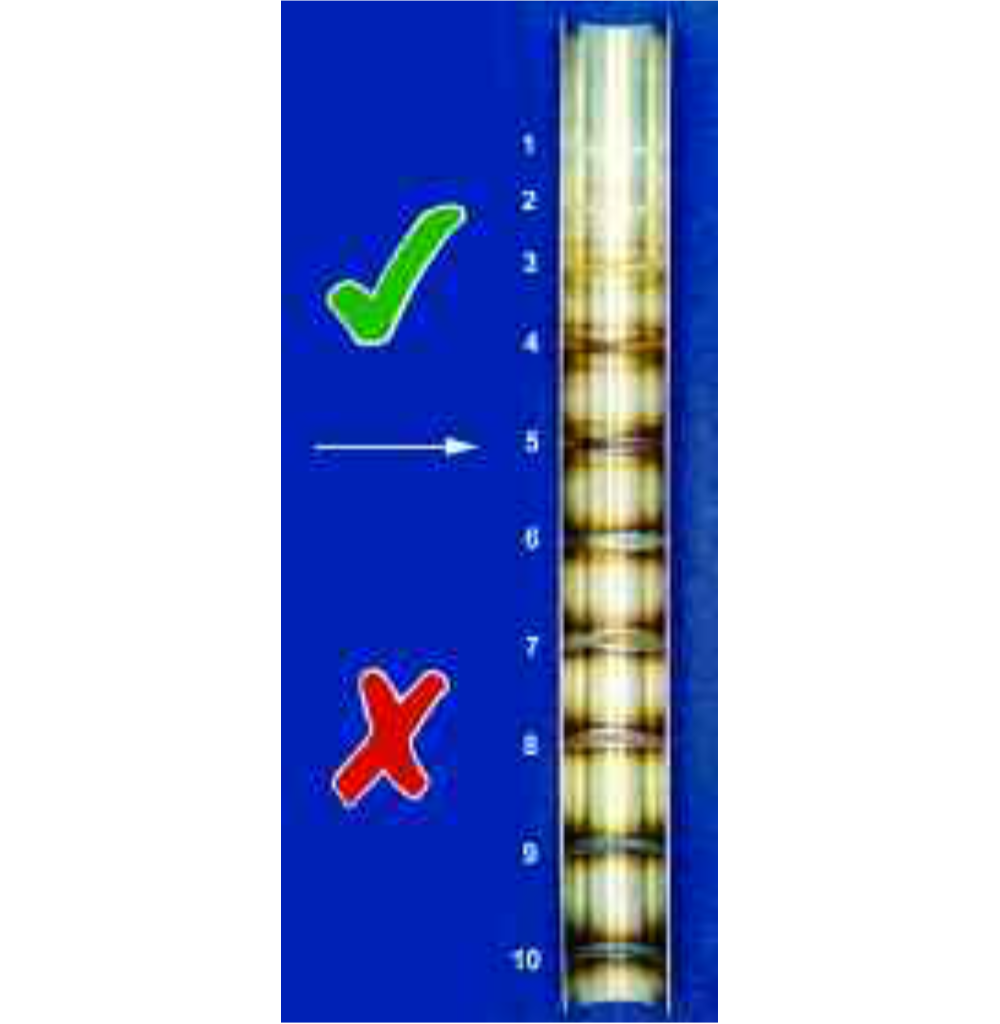
Although not strictly necessary after pickling, a finished component may be 'passivated'. This involves immersion of small components, or the flushing through of pipework, with nitric acid. This does not corrode the stainless steel nor remove the surface layer, but it helps to thicken, and so strengthen, the passive layer.
The techniques appropriate to the post-fabrication clean-up of stainless steel components and, particularly, their preparation for first use as food processing equipment, are well documented [10, 11].
6 Surface finish
The rougher a surface is, the more easily matter such as food will stick to it. This food will harbour micro-organisms which, if they are not removed, may multiply and cross-infect the next batch of food. And the rougher a surface is, the more difficult it will also be to clean. A smooth surface, durable enough to resist cracking, chipping, flaking and abrasion can not only resist the build-up of process soils but also be easily cleaned and disinfected.

It is not always easy to quantify the roughness of a surface. The usual technique is to draw a diamond stylus across the material and record its deflections as it follows the irregularities of the surface. 'Roughness' is then calculated from the deviations of the stylus from an arbitrary centre line and expressed as an Ra value [12]. Unfortunately, the size (and thus the sensitivity) of the diamond tip is many times larger than the size of a micro-organism, and fine surface irregularities which are still deep enough to harbour micro-organisms will not be detected. In addition, if a surface includes a wide range of roughness- es along the length sampled, the calculations will simply average them out, perhaps concealing the existence of a small, but particularly rough, region within the area studied.

Nevertheless, as a guide as to whether or not a surface is smooth enough to resist accumulating process soils and to be easy to clean, EHEDG 6 Document No. 8 [13] recommends that "large areas of product contact surface should have a surface finish of 0.8 μm Ra or better". Cold-rolled stainless steel up to 4 mm thick has a roughness of the order of Ra = 0.2 μm to 0.5 μm and so it does not usually need to be polished, provided that the fabrication processes themselves do not create areas rougher than 0.8 μm Ra. Sheet thicker than 4 mm will have a roughness which is dependent on both the final thickness and the degree of cold reduction, and so it may not meet the target of 0.8 μm Ra and may require polishing before service. Welds, too, may have to be ground smooth to give them a surface finish close to that of the parent material.
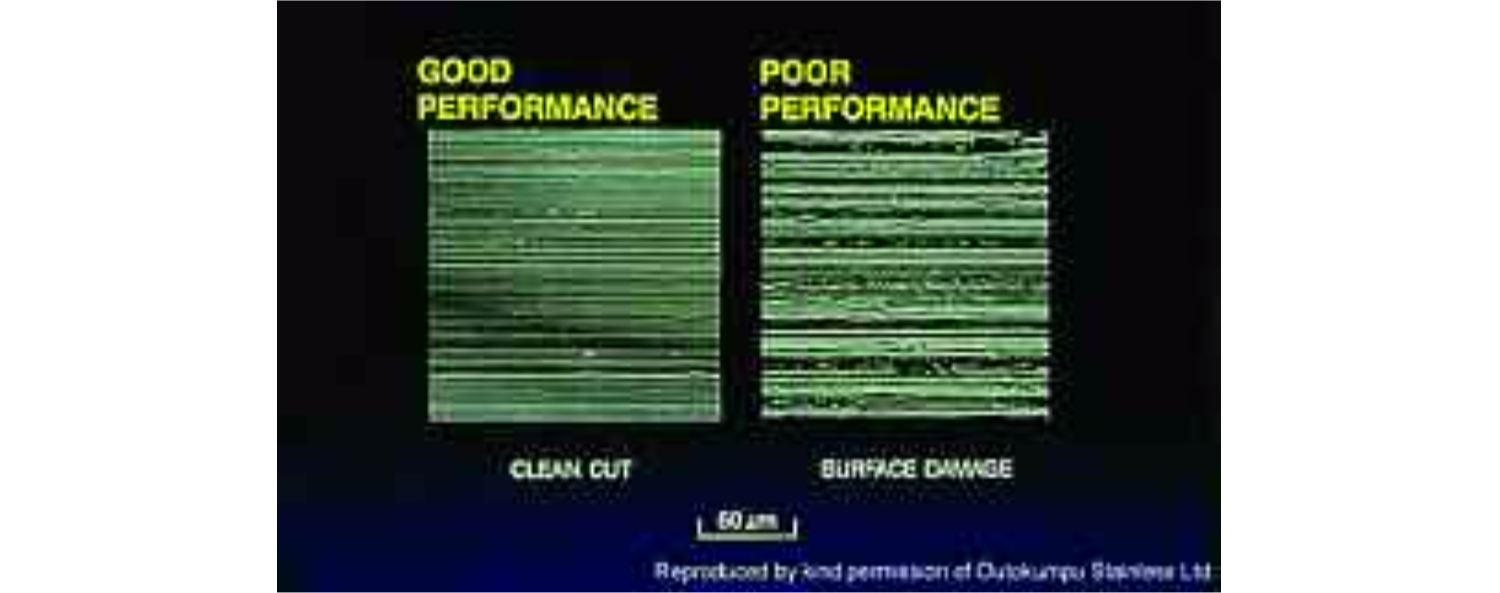
However, Ra values alone can be an unreliable indicator of the whether a surface will accumulate soils easily. The process by which a given surface finish has been created (by casting, turning, milling, mechanical polishing or shot-blasting, etc.) often has a greater influence on the rate at which contamination will build up on it than the surface's final Ra value itself. Some polishing techniques produce a clean-cut surface, as shown in the photomicrograph. Electropolishing can typically reduce the Ra value by half by reducing peaks (particularly small, sharp projections) and rounding them off. Other methods, however, can damage the surface. For instance, using an abrasive belt running at the wrong speed or applied at too great a pressure onto the workpiece can create tiny pits in the surface of the material or can tear it or can lift minute parts of the surface and fold them over, leaving sheltered 'caves' which can collect contamination which the cleaning processes cannot remove.
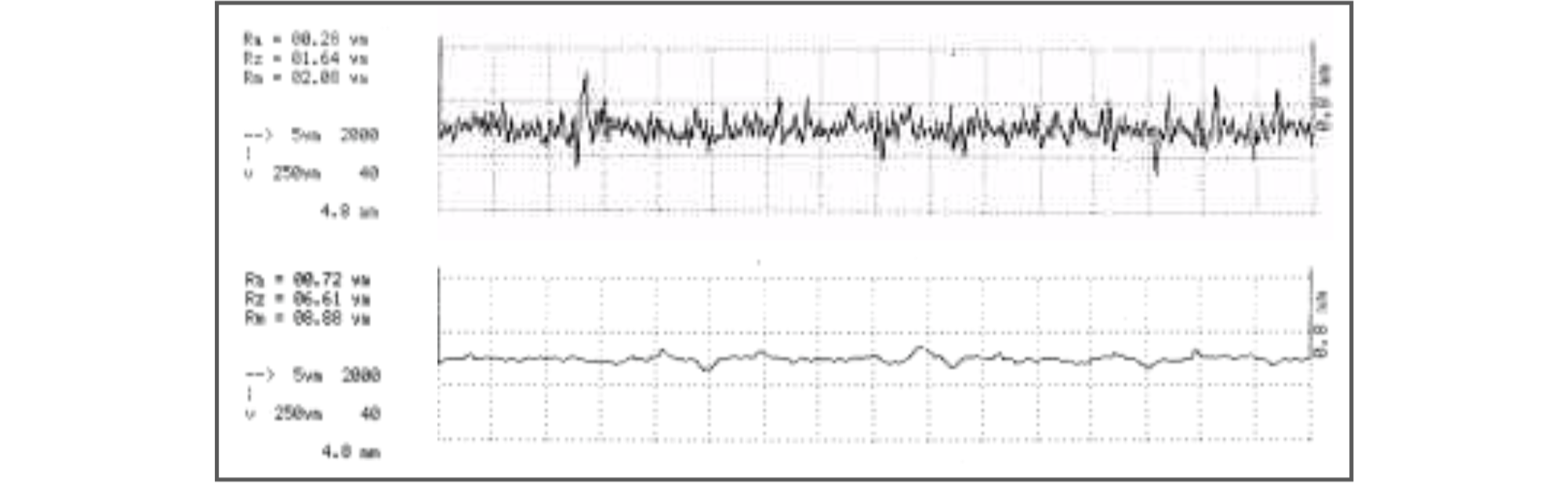
Surface finish is, of course, relatively easy to measure where the surface is accessible but it may not be so easily measured inside a length of pipe-work.
The efficiency with which any cleaning process can remove accumulated contamination is also strongly influenced by the way it is performed. Cleaning depends upon the combined effects of chemical action, mechanical action, temperature and time, and so if the velocity with which a detergent passes across a surface is increased, this may allow its concentration or its temperature or its contact time to be reduced. Alternatively, if a rough surface cannot be made smoother, perhaps because of its inaccessibility, then increasing the velocity of the detergent may mean that it can still be effectively cleaned with proprietary chemicals at their recommended concentrations and temperature and within the normal time. EHEDG document No. 17 [14], for instance, advises that: "higher liquid velocities may allow surfaces with a roughness above Ra = 0.8 μm to clean acceptably up to a roughness of Ra = 3.2 μm".

Whatever the technique employed to prepare a surface or the procedure used to clean it after use, it is important to confirm the surface's cleanability under service conditions.
7 Design principles
The demands upon the design of equipment intended for the processing or storage of foods and beverages are stringent. The EC Machinery Directive 98/37/EC: 1998 [15] states that machinery "must be so designed and constructed as to avoid any risk of infection, sickness or contagion". EN 1672-2: 2005 [16] demands that it is "capable of being properly operated, cleaned and maintained". Extensive advice on how to meet these requirements is available to the designer and specifier from the European Hygienic Engineering & Design Group, a consortium of equipment manufacturers, food industries, research institutes and public health authorities founded in 1989 to promote hygiene during the processing and handling of food products.
Many of the features of a design which make it 'hygienic' are the same as those which will help to reduce corrosion, and so the inherent corrosion resistance of a material like stainless steel is reinforced by hygienic design. It should be noted that 'hygienic' does not necessarily mean 'bacteria-tight'; hygienic design and construction will render the equipment non-contaminating and cleanable, but cannot not guarantee sterility.
All drawings: Nickel Institute
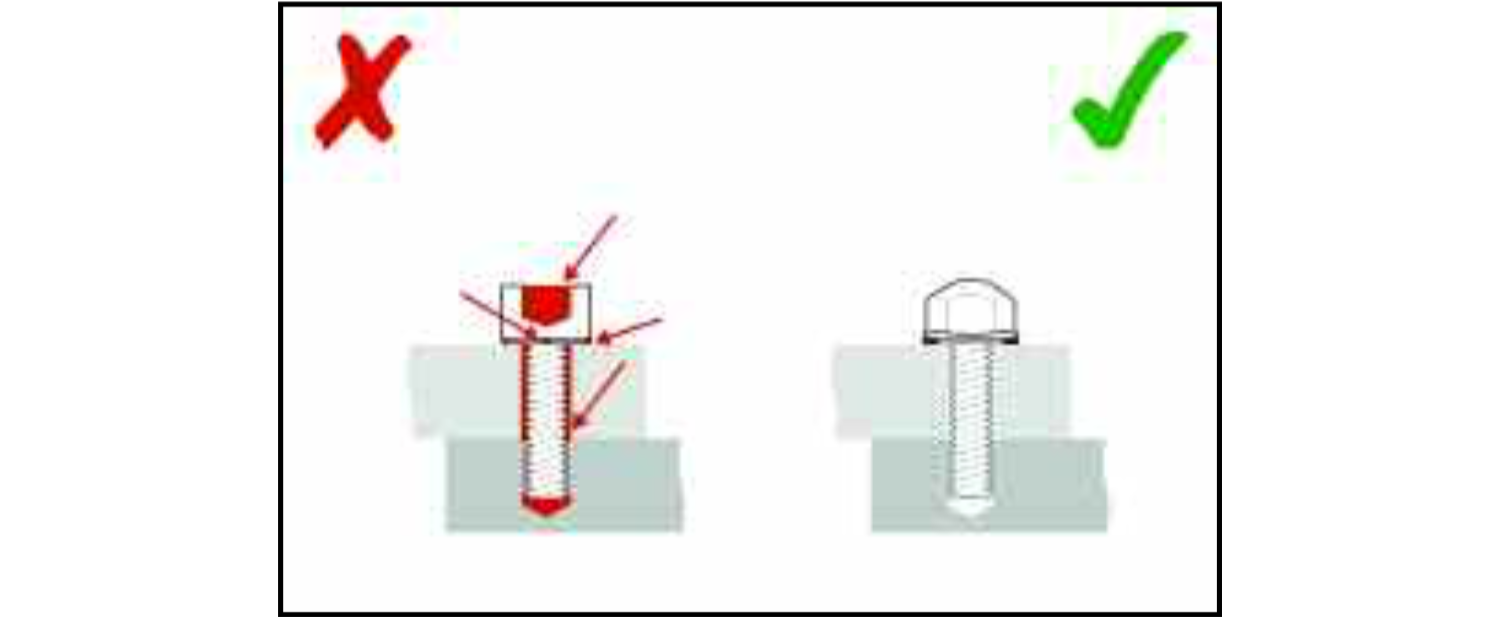
If a design leaves crevices between components, for instance under the heads of bolts or at flanged joints, these will not only retain process soils but also be very difficult to clean. Cleaning agents may also be trapped in such crevices and, because they can then be held in contact with the material of the equipment for a longer period of time was intended, they can cause corrosion inside the crevice.
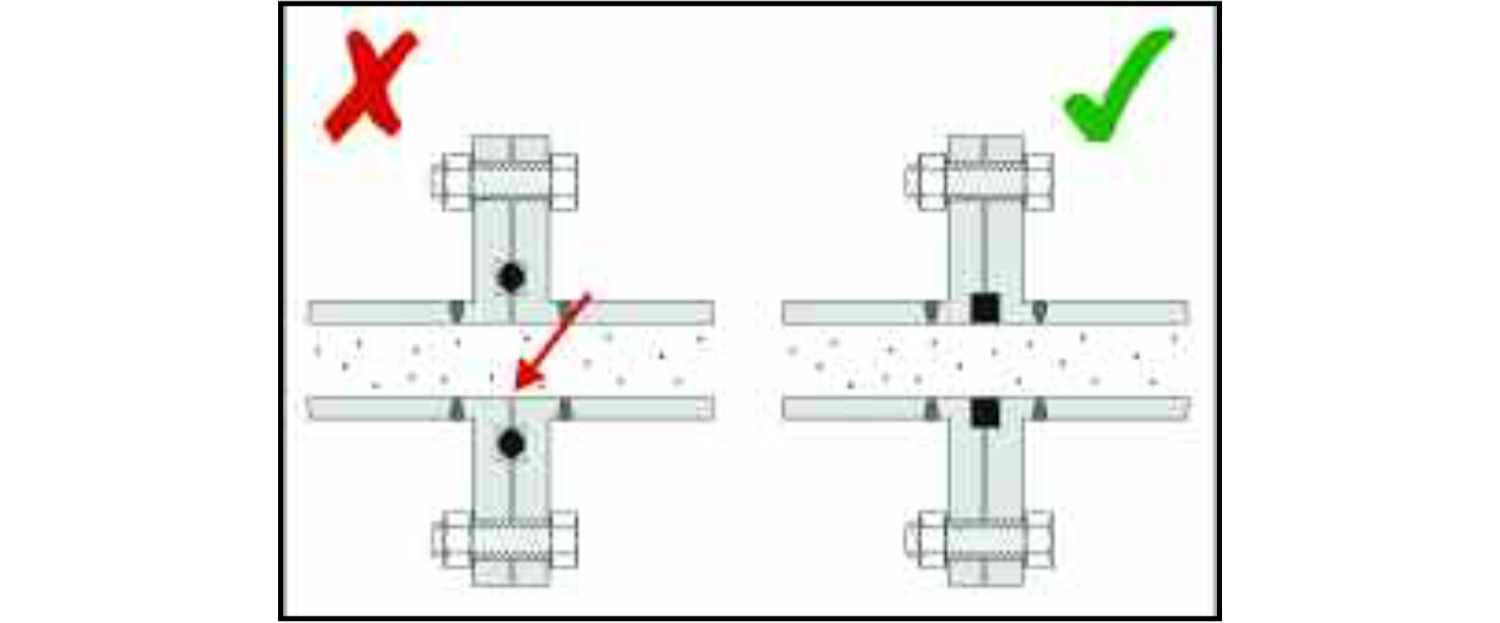
Furthermore, because the conditions inside a crevice are quite different from those on an open surface, an effect known as crevice corrosion will accelerate the corrosion process. On an open stainless steel surface accessible to oxygen, damage caused by, for instance, chlorides will generally be quickly repaired, but inside a crevice where oxygen is not easily replaced once it has been used up by the healing process, the chlorides will attack the material rapidly.
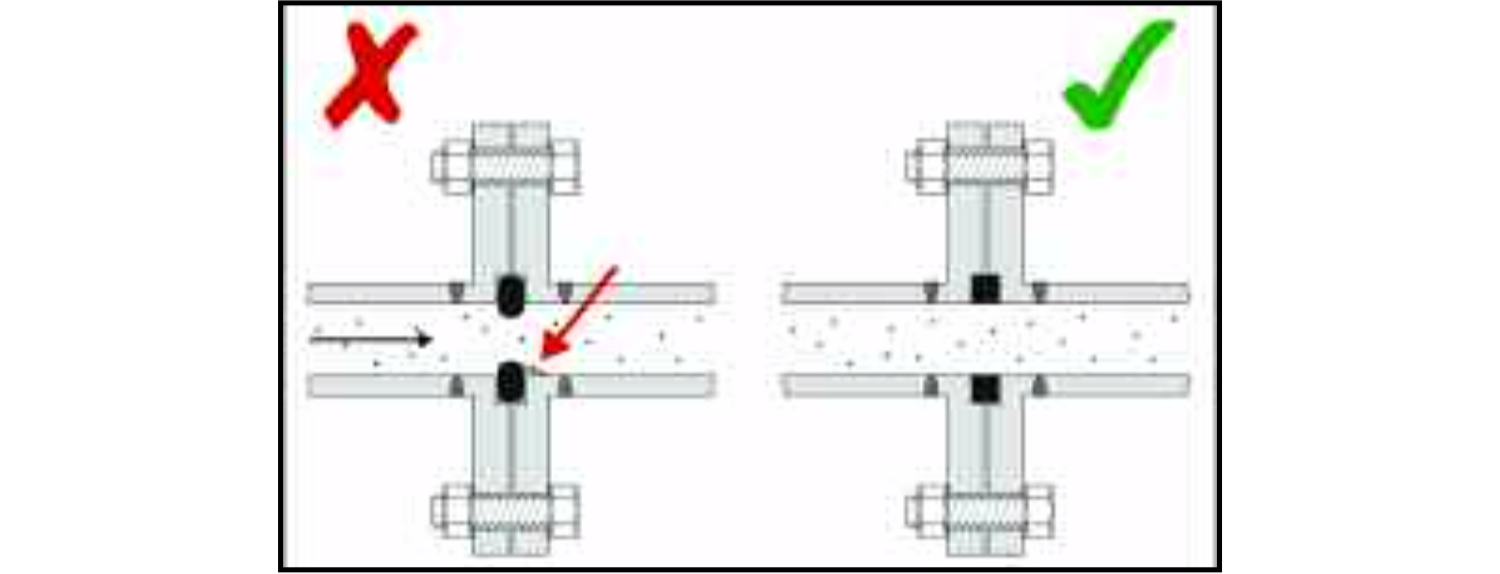
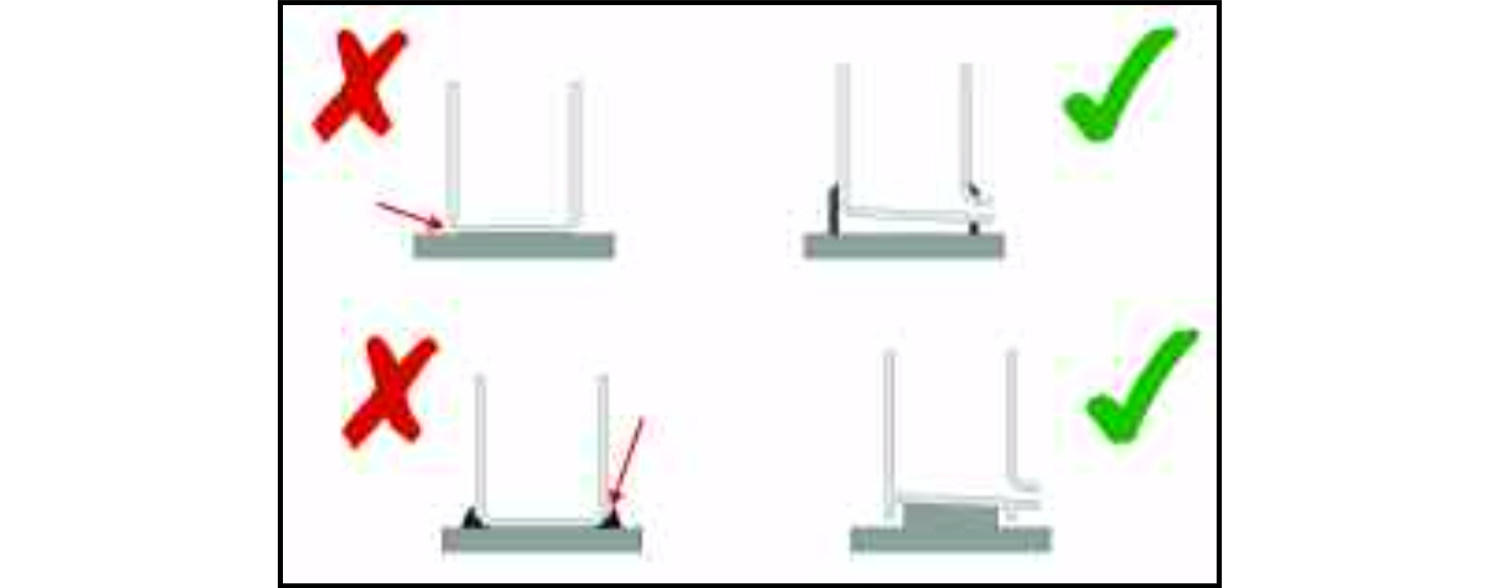
The more smoothly the food product can flow through the equipment, the less danger there is of it being trapped in a 'dead area' which can be difficult to clean. A dead area can be created if a synthetic seal at a joint protrudes into the flow of the product.
This may happen when the two faces of the joint are clamped together, compressing the seal, or because it expands when it becomes warm. In either case, the projection of the seal may cause a small proportion of the food to be trapped on the downstream side of the obstruction instead of being carried along the pipe with the remainder of the food. A hygienic design will ensure that when the joint is correctly clamped together and the equipment is at the normal operating temperature, the seal is just level with the bores of the two sections of pipe-work.
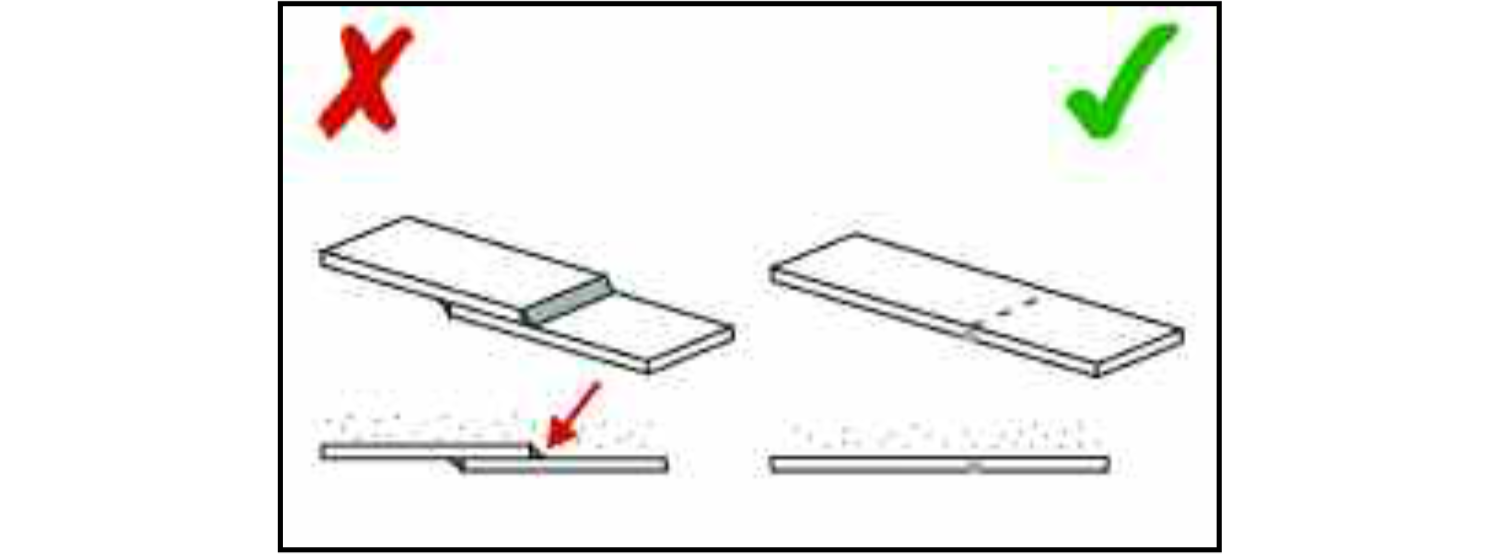
A lap joint at a weld will create a step between two surfaces. This can retain food which will remain there and deteriorate until it is removed by the cleaning processes. A butt joint, ground smooth after welding, is far superior both because it will not allow the accumulation of process soils and because its smoother surface is easier to clean. It must, of course, be continuous along the length of the joint. Non-metallic fillers are not a satisfactory alternative to a continuous weld as they can, in time, develop cracks or separate away from the metal, revealing a narrow crevice which will be very difficult to keep clean.
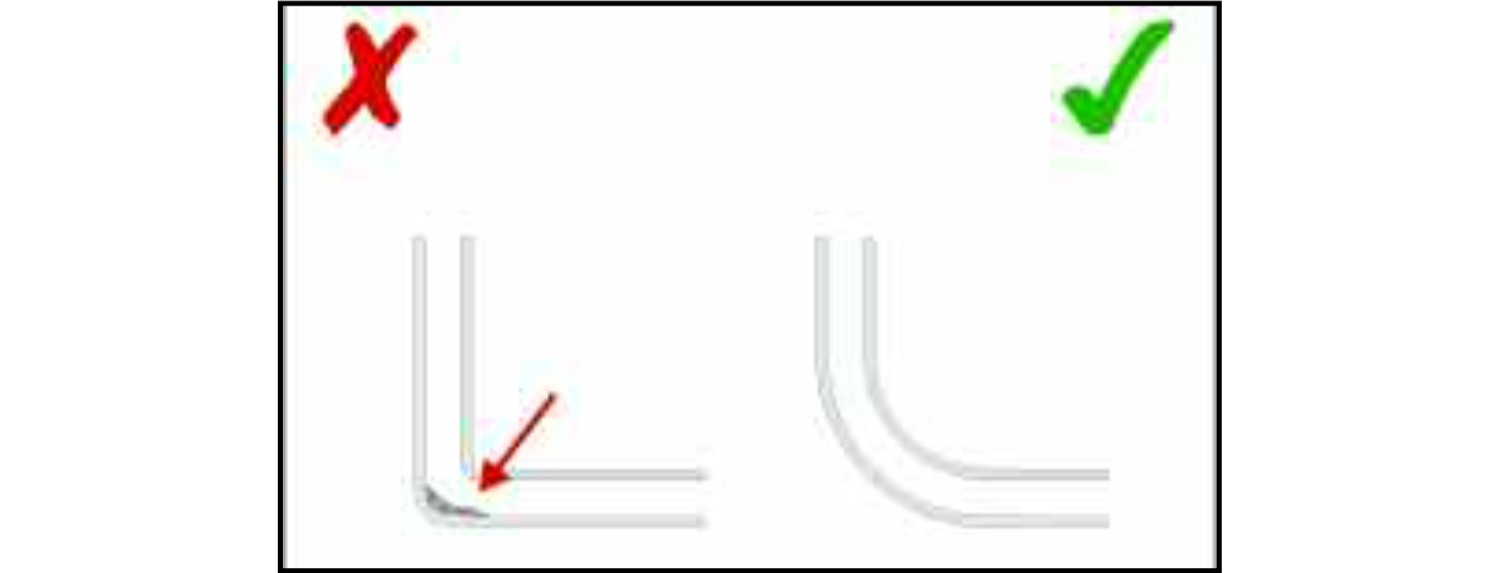
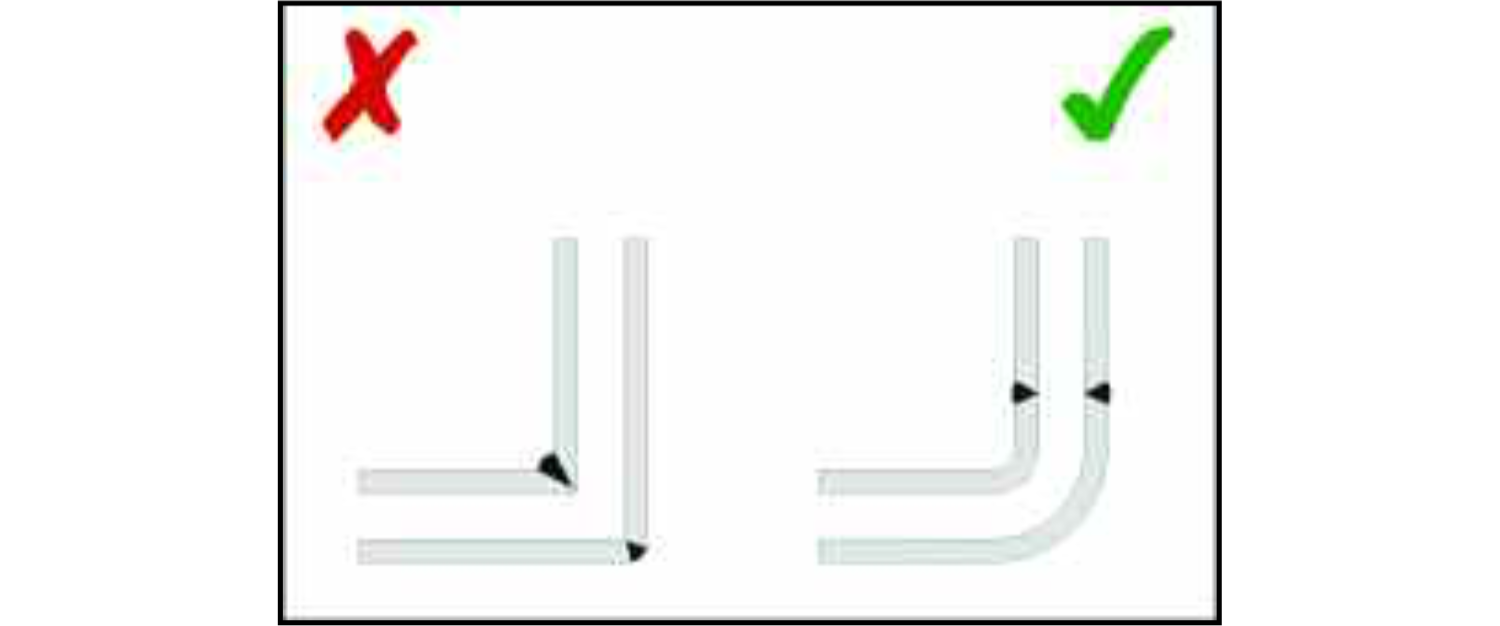
Where there is a junction between two pipes a smooth bend in the pipework and a weld on a straight section of the pipe above the bend is preferable to a butt joint. This design feature will help to ensure that liquid foods can drain away from the weld rather than being retained in any irregularities on its surface. The preferred method for joining pipework is automatic orbital welding which is capable of consistently high quality welds.
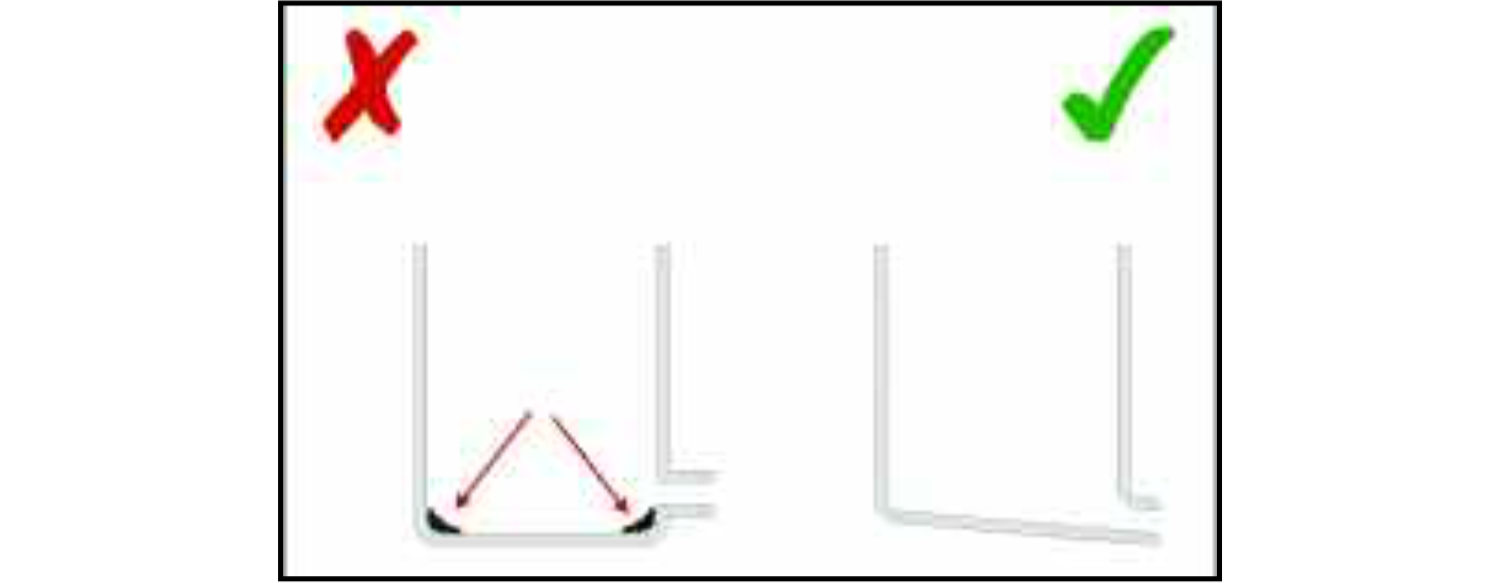
Pipe-work itself should present as little restriction to the smooth flow of the product as possible. Tight bends can trap foods. The larger the radius of a bend the more freely the product will flow and the less hygiene risk there will be. The same applies to the corners of vessels in which the product may be stored — the larger the radii of the corners, the easier they are to clean. EHEDG Document 10 [17] recommends that corners of closed equipment (where access for cleaning can be very difficult) have a minimum radius of 3 mm.
Vessels in which food is stored should drain naturally — there should be no corners in which it can collect. EHEDG Document 13 [18] recommends that the minimum slope of the bottom of a tank is 3° from the horizontal. This, of course, assumes that the product is sufficiently liquid to be self-draining, given that 3° slope — if it is a thick sauce or a paste it may require a greater slope in order to ensure that it does not accumulate on the internal surfaces of the vessel.
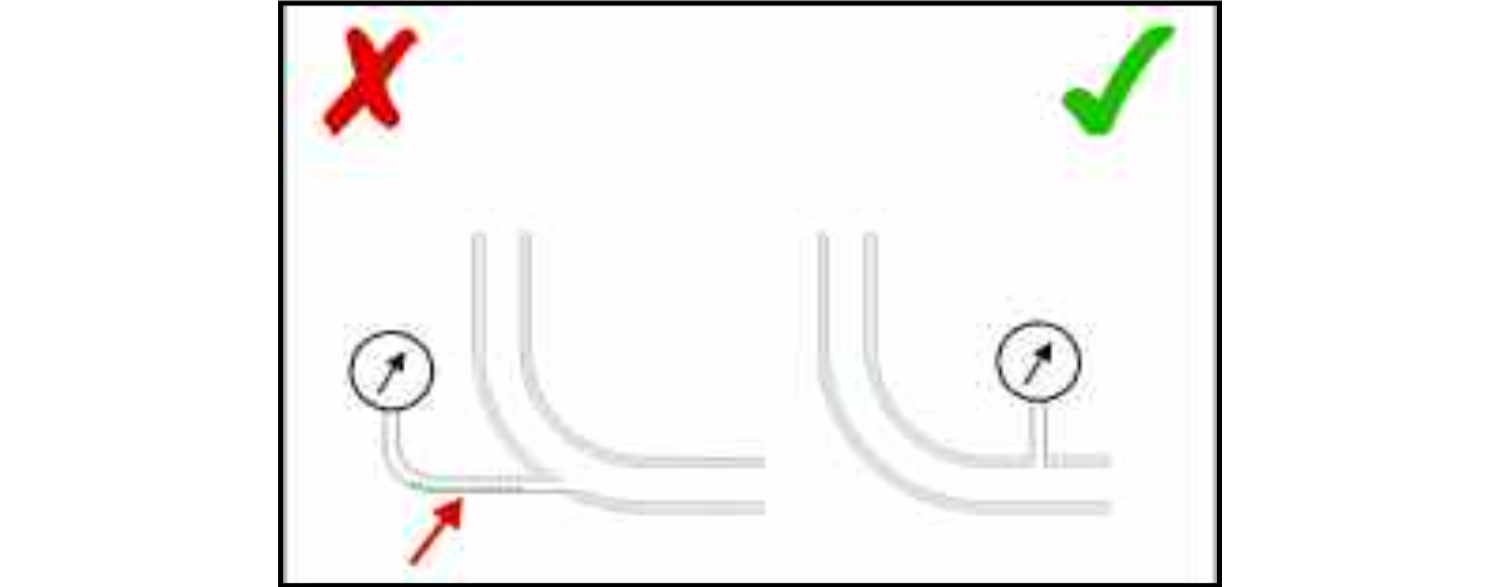
When instruments are incorporated into a design, they will inevitably create areas in which foods might be trapped, but if they are designed correctly the risk can be significantly reduced. Ideally, positioning the arm in which the instrument is to be mounted above the flow of the food product will minimise the risk of food entering it.
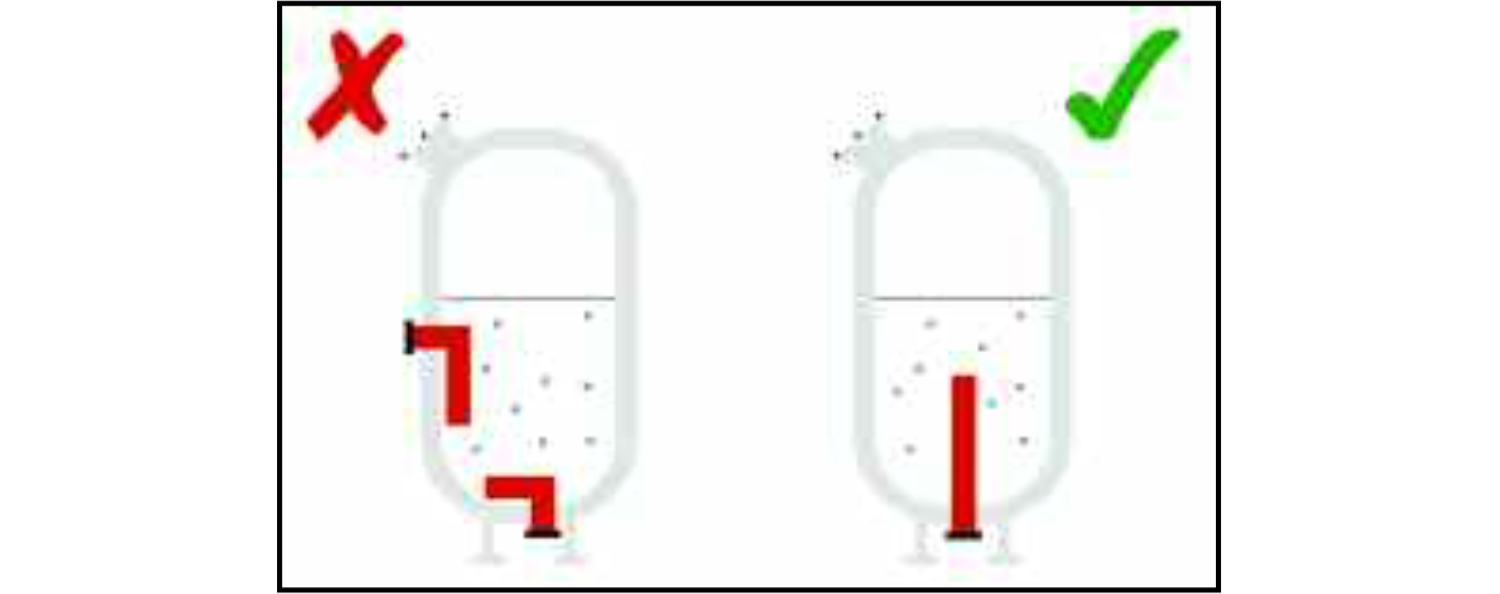
Although a system may be designed to operate at a temperature where corrosion would not normally be expected, localised conditions may be quite different from the design 'average' temperature. For instance, a vessel under the stress of an internal pressure and warming a fluid containing chlorides may not be expected to suffer from stress-corrosion cracking if the temperature is intended to remain low (well below about 55°C). However, around the heating elements themselves the temperature of the fluid may well exceed this, and if a heating-element is close to the wall of the vessel, the stainless steel in that area may suffer from this form of corrosion.
It is also important to design against corrosion from the outside of a vessel. If the chemicals used to clean the exterior sur- faces of fixed tanks can be retained in crevices between the tank and the surface on which it is mounted, corrosion may occur by the same mechanism as described above for bolts and flanges. A tank resting on a concrete base will create just such a crevice. Grouting this crevice may initially seal it, but grout can shrink and crack with time, recreating crevices which can lead to corrosion. Raising the tank off the floor by mounting it on legs eliminates the crevice. Alternatively, the tank could be supported on a plinth within its base and be fitted with a drip-skirt all the way round to protect the crevice between the tank and the plinth from the cleaning agents.
8 Conclusion
Apart from their pleasing appearance, stainless steels have many other attributes essential to the hygienic preparation of foods and beverages. They are inert in most foods, they are easily fabricated, they are durable and, when components become obsolete, the material is recyclable. The correct grade of steel for an application, designed into equipment with hygiene in mind and prepared with care will offer many years of reliable service and so represent an excellent investment.
9 European standards
Food processing machinery:
- EN 1672-2; Food processing machinery — Basic concepts — Part 2: Hygiene requirements.
- EN 13732; Food processing machinery — Bulk milk coolers on farms — Requirements for construction, performance, suitability for use, safety and hygiene.
Tubes and pipes:
- EN 12502-4; Protection of metallic materials against corrosion — Guidance on the assessment of corrosion likelihood in water distribution and storage systems — Part 4: Influencing factors for stainless steels.
Drinking water installations:
- EN 10312; Welded stainless steel tubes for the conveyance of aqueous liquids including water for human consumption — Technical delivery conditions.
Cutlery and tableware:
- EN 8442-1; Materials and articles in con- tact with foodstuffs — Cutlery and table holloware — Part 1: Requirements for cutlery for the preparation of food.
- EN 8442-2; Materials and articles in contact with foodstuffs — Cutlery and table holloware — Part 2: Requirements for gold-plated cutlery.
10 References
[1] European Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to come into Contact with Food and repealing Directives 80/590/EEC and 89/109/EEC. Official Journal of the European Communities L 338, 13/11/2004, 4-14 2004.
[2] Decreto ministeriale 21 marzo 1973: Disciplina igienica degli imballaggi, recipienti, utensili, destinati a venire in contatto con le sostanze alimentare o con sostanze d’uso personale. Supplemento ordinario alla “Gazzetta ufficiale” della Repubblica Italiana n. 104 del 20 aprile 1973 [Regulations on the hygiene of packaging, recepta- cles and tools intended to come into contact with substances for food use or with sub- stances for personal use. Ministerial Decree 21 March 1973. Official Gazette of the Italian Republic no. 104 of 20 April 1973]; in Italian only.
[3] FAILLE, C., MEMBRE, J. M., TISSIER, J. P., BELLON-FONTAINE, M. N., CARPENTIER, B., LAROCHE, M. A. and BENEZECH, T., “Influence of physiochemical properties on the hygienic status of stainless steel with various finishes”, Biofouling 15, 261-274, 2000.
[4] TUTHILL, A. H. and COVERT, R. A., Stainless steels: an introduction to their metallurgy and corrosion resistance (Nickel Institute publication 14 056), Toronto 2000†.
[5] Stainless Steel: Tables of Technical Properties (Materials and Applications Series, Volume 5), Luxembourg: Euro Inox 2005.
[6] Welding of stainless steels and other joining methods (A Designer's Handbook Series No. 9 002), AISI, Washington, D.C. 1979†.
[7] Guidelines for the welded fabrication of nickel-containing stainless steels for corrosion resistant services (NiDI Reference Book Series No. 11 007) Toronto: Nickel Institute 1992†.
[8] Welding stainless steel to meet hygienic requirements (Guideline Document 9), Brussels: EHEDG 1993ø
[9] Specification for welding of austenitic stainless steel tube and pipe systems in sanitary (hygienic) applications (AWS D18.1), Miami: American Welding Society 1999.
[10] TUTHILL, A. H., Fabrication and post-fabrication cleanup of stainless steels (NiDI Technical Series No. 10 004), Toronto 1986†.
[11] TUTHILL, A. H., AVERY, R. E., and COVERT, R. A., Cleaning stainless steel surfaces prior to sanitary service (NiDI Technical Series No. 10 080), Toronto 1997†.
[12] Geometrical product specifications – surface texture: profile method – terms, definitions and surface texture parameters (ISO 4287), 1997.
[13] Hygienic equipment design criteria (Guideline Document No. 8), Brussels: EHEDG 2004ø.
[14] Hygienic design of pumps, homogenisers and dampening devices (Guideline Document No. 17), Brussels: EHEDG 1998ø.
[15] The Machinery Directive: European Community Directive 98/37/EC (1998), relating to Machinery (Official Journal of the European Communities L 207, 1–46) 1988.
[16] EN 1672-2: 2005 Food Processing Machinery. Basic Concepts. Part 2: Hygiene requirements 2005.
[17] Hygienic design of closed equipment for the processing of liquid food (Guideline Document No. 10), Brussels: EHEDG 2004ø.
[18] Hygienic design of equipment for open processing (Guideline Document No. 13), Brussels: EHEDG 2004ø.
ø also available online at www.ehedg.org
† also available online at www.nickelinstitute.org
11 Where to go for help
EHEDG: www.ehedg.org
Nickel Institute: www.nickelinstitute.org
12 Appendix
Table 1. The designations of the stainless steels referred to in this brochure.
| Group | EN | AISI |
|---|---|---|
| Martensic | 1.4021 | 420 |
| 1.4116 | ||
| 1.4125 | 440C | |
| Ferritic | 1.4016 | 430 |
| 1.4509 | 441 | |
| 1.4510 | 439 | |
| 1.4521 | 444 | |
| Austenitic | 1.4301 | 304 |
| 1.4307 | 304L | |
| 1.4401 | 316 | |
| 1.4404 | 316L | |
| 1.4541 | 321 | |
| Super-austenitic | 1.4539 | 904L |
| 1.4547 | ||
| 1.4529 | ||
| Precipitation-hardening | 1.4542 | 630 |
| Duplex | 1.4462 | |
| 1.4362 |
Table 2. Typical compositions of the stainless steels referred to in this brochure.
| EN | AISI | C% | Cr% | Ni% | Mo% | N% | Cu% | Others |
|---|---|---|---|---|---|---|---|---|
| min-max | min-max | min-max | min-max | min-max | min-max | |||
| 1.4021 | 420 | 0.16 - 0.25 | 12 - 14 | |||||
| 1.4116 | 0.45 - 0.55 | 14 - 15 | 0.5 - 0.8 | V% = 0.10 to 0.20 | ||||
| 1.4125 | 440C | 0.95 - 1.20 | 16 - 18 | 0.4 - 0.8 | ||||
| 1.4016 | 430 | 008 | 16 - 18 | |||||
| 1.4509 | 441 | 0.03- | 17.5 - 18.5 | NB% = 3xC%+0.30 to 1.00 Ti% = 0.10 to 0.60 | ||||
| 1.4510 | 439 | 0.05 | 16 - 18 | Ti% = 4x(C%+N%)+0.15 to 0.80 | ||||
| 1.4521 | 444 | 0.025 | 17 - 20 | 1.8 - 2.5 | 0.030 | |||
| 1.4301 | 304 | 0.07 | 17 - 19.5 | 8 - 10.5 | 0.11 | |||
| 1.4307 | 304L | 0.030 | 17.5 - 19.5 | 8 - 10 | 0.11 | |||
| 1.4401 | 316 | 0.07 | 16.5 - 18.5 | 10 - 13 | 2.0 - 2.5 | 0.11 | ||
| 1.4404 | 316L | 0.030 | 16.5 - 18.5 | 10 - 13 | 2.0 - 2,5 | 0.11 | ||
| 1.4541 | 321 | 0.08 | 17 - 19 | 9 - 12 | Ti% = 5xC% to 0.7 | |||
| 1.4539 | 904L | 0.020 | 19 - 21 | 24 - 26 | 4.0 - 5.0 | 0.15 | 1.2 - 2.0 | |
| 1.4547 | 0.020 | 19.5 - 20.5 | 17.5 - 18.5 | 6.0 - 7.0 | 0.18 - 0.25 | 0.5 - 1.0 | ||
| 1.4529 | 0.020 | 19 - 21 | 24 - 26 | 6.0 - 7.0 | -.15 - -0.25 | 0.5 - 1.5 | ||
| 1.4542 | 630 | 0.07 | 15 - 17 | 3 - 5 | 0.6 | 0.45 | 3 - 5 | Nb% = 5xC% to 0.45 |
| 1.4462 | 0.030 | 21 - 23 | 4.5 - 6.5 | 2.5 - 3.5 | 0.10 - 0.22 | |||
| 1.4362 | 0,030 | 22 - 24 | 3.5 - 5.5 | 0.1 - 0.6 | 0.05 - 0.2- | 0.1 - 0.6 |